
Book a 30-minute Strategy Consultation
Schedule a meeting with one of our Medical Experts to discuss your CDP and Trial Design
Inovia's Guide to CDP and Trial Design Success
Less than 10% of novel compounds that enter phase 1 clinical trials will obtain regulatory approval. A well-designed and thought-through Clinical Development Plan provides a comprehensive strategy for developing an asset through regulatory submission and gives you the best chances of regualtory approval .
At Inovia Bio we using our propetiary technolgoy and medical expertise we have developed a formual that maximises accuracy and speed.
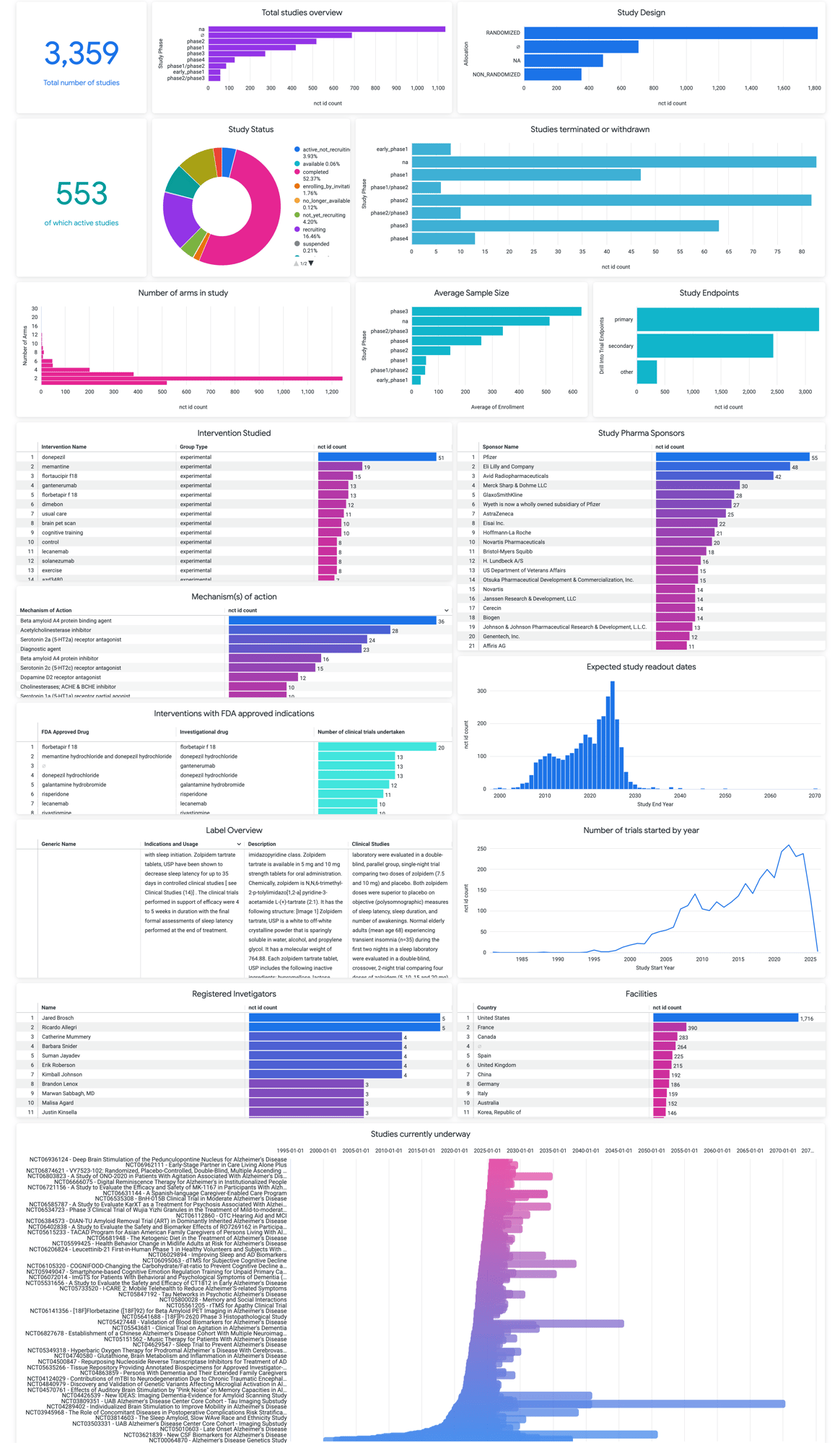
We analyse early data on the drug’s pharmacology, toxicology, and safety for preclinical studies to determine strategy for clinical trials
Utilising InovaLandscape and Medical Expertise, we identify the target patient population, disease, and outcomes. InovaLandscape provides Real-time information on current and previous trials and the clinical outcomes used.
Combining our Medical Experts and InovaLandscape, we analyse the most effective endpoints to use, the inclusion and Exclusion criteria and subject numbers
We define what constitues proof of concept for effiacy, aiding Go/NoGo decisions.
We believe in designing trials to suit our partners' budgets and resources, with strictly no change orders. Using InovaLandscape we track competitor timelines, allowing you to make informed decisions.
We strongly believe in identifying risks and developing mitigation strategies, with multiple different strategies. This is enhanced with our preferred early adoption of an Integrated Evidence Plan, using InovaIEP
In order to create a CDP that provides a multi-layer strategy, we promote involving multiple functions, such as Clinical Development, Regulatory, Medical Affairs, Clinical Operations and Biostatistics.

This tool will automatically generate your study diagram and a schedule of assessments table, which can be exported directly into a protocol.

Schedule a meeting with one of our Medical Experts to discuss your CDP and Trial Design